Terpenes and flavonoids, two prominent classes of secondary metabolites abundant in Cannabis sativa, are emerging as promising candidates in the therapeutic landscape of diabetes mellitus and its associated complications, including diabetic neuropathy. Given the World Health Organization’s projection that over 800 million individuals worldwide are affected by diabetes, the exploration of these phytochemicals offers a compelling avenue for the development of cost-effective and accessible adjunct or alternative therapies. Their multifaceted pharmacological profiles—encompassing antioxidant, anti-inflammatory, and neuroprotective properties—underscore their potential to address key pathophysiological mechanisms underpinning diabetic disorders.
[Editor’s Note: all information in this article is derived from The Big Book of Terps Edition 2, and The Formidable Book of Flavonoids, by Russ Hudson at cannabischemistry.org.]
DIABETES DEFINED
Diabetes mellitus is a chronic metabolic disorder characterized by dysregulated glucose homeostasis resulting from impaired insulin secretion, insulin action, or both. This disease encompasses a spectrum of etiologically distinct subtypes, primarily type 1 (autoimmune β-cell destruction) and type 2 (insulin resistance with relative β-cell dysfunction), leading to chronic hyperglycemia and associated microvascular and macrovascular complications.
CONSEQUENCES OF DIABETES
Diabetes precipitates a broad spectrum of pathophysiological sequelae attributable to chronic hyperglycemia and metabolic dysregulation. Prolonged glycemic imbalance engenders endothelial dysfunction, oxidative stress, and low-grade systemic inflammation, culminating in microvascular complications such as retinopathy, nephropathy, and neuropathy. Concomitantly, it accelerates atherogenesis, markedly increasing the risk of macrovascular events including myocardial infarction, stroke, and peripheral arterial disease. Additionally, impaired immune competence and poor wound healing contribute to heightened susceptibility to infection and increased morbidity. These complications collectively result in significant reductions in quality of life, functional capacity, and overall life expectancy.
TERPENES DEFINED
Terpenes are a diverse class of naturally occurring organic hydrocarbons constructed from isoprene (C₅H₈) units via the mevalonate or methylerythritol phosphate pathways. They are biosynthetically classified based on the number of isoprene units, ranging from monoterpenes (C₁₀) to polyterpenes (C₄₀+), and serve critical roles in primary and secondary metabolism, including membrane structure stabilization (e.g., sterols) and ecological interactions such as pollinator attraction and herbivore deterrence.
Terpenoids, also known as isoprenoids, represent oxygenated derivatives or structurally modified forms of terpenes, incorporating functional groups such as alcohols, ketones, aldehydes, or epoxides. These structural modifications confer enhanced chemical reactivity and biological activity, underpinning their widespread pharmacological and industrial applications. While all terpenoids are derived from terpenes, not all terpenes possess the oxygenation characteristic of terpenoids.
FLAVONOIDS DEFINED
Flavonoids are a large and structurally diverse class of polyphenolic secondary metabolites ubiquitously distributed in the plant kingdom. They are biosynthetically derived from the phenylpropanoid and polyketide pathways, forming a common C6–C3–C6 carbon skeleton composed of two aromatic rings (A and B) connected by a three-carbon bridge, often forming a heterocyclic C-ring. Flavonoids are subclassified into flavones, flavonols, flavanones, flavanols (catechins), isoflavones, and anthocyanidins based on the oxidation state and functionalization of the central C-ring.
Functionally, flavonoids play essential roles in plant physiology, including UV filtration, pigmentation, pathogen defense, and symbiotic nitrogen fixation. In human health, they exhibit pleiotropic bioactivities such as antioxidant, anti-inflammatory, anti-carcinogenic, and cardioprotective effects, primarily through modulation of cell signaling pathways, enzyme activity, and gene expression. Their therapeutic potential is mediated by their ability to scavenge reactive oxygen species, chelate metal ions, and modulate key molecular targets involved in chronic disease pathogenesis.
TERPENES AND FLAVONOIDS IN CANNABIS
Terpenes and flavonoids are integral to the phytochemical complexity and pharmacological potential of Cannabis sativa, encompassing both drug-type (high-THC) and hemp-type (low-THC) chemovars. These secondary metabolites not only contribute to the organoleptic properties—aroma, flavor, and pigmentation—of the plant, but also modulate its therapeutic efficacy through synergistic interactions known as the “entourage effect” when occurring within the endocannabinoid system (ECS), and “synergy” both within and outside of the ECS.
Terpenes exhibit a range of pharmacodynamic actions, including analgesic, anxiolytic, anti-inflammatory, and antimicrobial effects, and are differentially expressed across chemovars, contributing to the heterogeneity in clinical outcomes.
Flavonoids, particularly the cannabis-specific subclass known as “cannaflavins” (e.g., cannaflavin A, B, and C), are less abundant but highly bioactive constituents. Synthesized via the phenylpropanoid pathway, these compounds display potent anti-inflammatory and neuroprotective properties, with some exhibiting non-cannabinoid-mediated inhibition of prostaglandin E2 production.
Both flavonoid and terpene expression are influenced by genotype, environmental conditions, and cultivation practices, leading to significant chemotypic variation within and between cannabis subspecies. Both molecules have potential to treat diabetes and related conditions as detailed below:
BETA-CARYOPHYLLENE – BICYCLIC SESQUITERPENE
β-Caryophyllene demonstrates significant therapeutic potential in the management of diabetes mellitus. Empirical evidence indicates its capacity to markedly attenuate hyperglycemia while concurrently enhancing endogenous insulin secretion[1]. Notably, its pharmacodynamic profile suggests superior efficacy and a more favorable side effect spectrum compared to pioglitazone[2], a widely prescribed thiazolidinedione.
NEROLIDOL – SESQUITERPENE
In a rat model of diabetes induced by a high-fat diet in conjunction with streptozotocin administration, nerolidol elicited a significant reduction in hyperglycemia and glycosylated hemoglobin levels, while concurrently enhancing body weight and circulating insulin concentrations. It notably improved the activity of carbohydrate-metabolizing enzymes and augmented hepatic glycogen storage. Additionally, nerolidol elevated serum triglyceride levels, significantly decreased total cholesterol, low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C) concentrations. Oxidative stress markers were attenuated, as evidenced by significant upregulation of superoxide dismutase and catalase activity, increased levels of reduced glutathione, and a marked reduction in thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxide concentrations, among various other antidiabetic effects[3].
MENTHOL – MONOTERPENE
Despite the well-documented health risks associated with menthol and tobacco, considerable biomedical interest persists regarding the therapeutic potential of this monoterpenoid. Numerous clinical investigations involving menthol are currently underway, including studies initiated in 2019 evaluating its efficacy in the management of painful diabetic peripheral neuropathy, its utility in general analgesia, and its potential role in modulating the reinforcing properties of nicotine among tobacco users, alongside a multitude of other ongoing clinical trials[4].
ORIENTIN – FLAVONE
Orientin, identified as the principal constituent in a methanolic extract derived from the leaves of Cecropia pachystachya (ambay pumpwood), demonstrated pronounced antihyperglycemic activity in alloxan-induced diabetic rats, with overall efficacy comparable to that of standard antidiabetic agents metformin and glibenclamide[5]. Similarly, when isolated as the major component of a hydroethanolic extract from the aerial parts of Parkinsonia (Cercidium), orientin significantly decreased serum and urinary glucose, urinary urea, and triglyceride levels in the same diabetic rodent model[6]. Additional findings from a separate study in rats revealed that Euterpe oleracea (açaí), a known natural source of orientin, modulates reactive oxygen species production by neutrophils and exerts a substantial protective effect on hepatic antioxidant defense mechanisms under oxidative stress, partially mitigating diabetes-induced hepatic damage[7]. Furthermore, in an in vitro model of mitochondrial dysfunction utilizing cultured skeletal muscle cells, orientin administration reduced intracellular reactive oxygen species production and upregulated the expression of genes implicated in mitochondrial function. The investigators concluded that orientin, along with structurally related flavonoids, possesses therapeutic potential comparable to established pharmacologic agents such as metformin and insulin in ameliorating mitochondrial dysfunction[8].
RUTIN – FLAVONOL
Rutin, both as a monotherapy and in combination with metformin, has been shown to elicit significant improvements in glycemic control, lipid profiles, and vascular reactivity in male Sprague Dawley rats, as evidenced by reductions in blood glucose, cholesterol, and triglyceride levels, attenuation of phenylephrine-induced vasoconstriction, and enhancement of acetylcholine- and sodium nitroprusside-induced vasorelaxation[9]. In both euglycemic and alloxan-induced diabetic rabbits, rutin pre-treatment restored neuronal and endothelium-dependent vasodilatory responses, thereby ameliorating endothelial dysfunction and nitrergic neuropathy[10].
As an antioxidant, rutin, when co-administered with rambutan honey—a product derived from the floral nectar of a tropical tree—significantly reduced plasma glucose levels and elevated insulin concentrations in streptozotocin-induced diabetic rats[11]. Furthermore, rutin has demonstrated potential in mitigating diabetes-associated cardiovascular pathology by reducing oxidative stress and reversing structural modifications in glycated low-density lipoprotein (LDL), the latter of which is strongly implicated in the pathogenesis of atherosclerosis among diabetic individuals[12].
Additionally, rutin extracted from Fagopyrum tataricum (tartary buckwheat) was found to improve glucose and lipid metabolism, attenuate colonic tissue damage, and modulate gut microbiota composition in diabetic murine models[13]. These findings underscore the emerging role of the gut microbiome in diabetes pathophysiology and therapeutic intervention. Finally, rutin appears to possess anti-atrophic properties relevant to diabetes-induced muscular degeneration, as evidenced by its ability to increase myocyte cross-sectional area and gastrocnemius muscle mass, while downregulating the expression of atrophy-associated proteins Atrogin-1 and MuRF1, thereby promoting muscle strength and mitigating sarcopenia[14].
LUTEOLIN – FLAVONE
In Wistar rats rendered type 2 diabetic through either a high-fat diet or streptozotocin administration, treatment with luteolin significantly mitigated dyslipidemia and improved the atherogenic index of plasma. Biochemically, luteolin administration resulted in elevated malondialdehyde levels and concomitant reductions in the antioxidant enzymes superoxide dismutase, catalase, and glutathione. At the molecular level, luteolin markedly upregulated peroxisome proliferator-activated receptor alpha (PPARα) expression—a key regulator of genes involved in lipid metabolism—while downregulating the expression of acyl-coenzyme A, cholesterol acyltransferase-2, and sterol regulatory element-binding protein-2, all of which are implicated in fatty acid and cholesterol biosynthesis. Moreover, luteolin effectively attenuated hepatic dysfunction, restoring liver morphology and function to near-normoglycemic conditions in diabetic rats[15].
VITEXIN – FLAVONE
Vitexin, identified as the principal bioactive constituent through guided fractionation of Ficus deltoidei (mistletoe fig), was demonstrated to significantly attenuate postprandial plasma glucose levels in normoglycemic mice subjected to a sucrose challenge[16]. In streptozotocin-induced diabetic mice—a model involving selective β-cell cytotoxicity—vitexin administration led to increased reproductive organ mass, amelioration of testicular histopathological damage, and significant modulation of key reproductive hormones, culminating in improved sexual behavior and fertility indices[17].
Additionally, vitexin has shown therapeutic promise in the management of diabetic nephropathy (DN). In rodent models of DN, vitexin alleviated renal fibrosis, structural injury, and ferroptosis, an iron-dependent form of regulated cell death, likely through activation of glutathione peroxidase 4 (GPX4), a critical enzyme in cellular defense against lipid peroxidation[18].
KAEMPFEROL – FLAVONOL
Kaempferol, isolated from Cuscuta pedicellata—a parasitic plant species—was shown to significantly reduce homeostasis model assessment-insulin resistance (HOMA-IR) and thiobarbituric acid reactive substances (TBARS) levels in rats with high-fat diet-induced obesity. These effects reflect a marked attenuation of insulin resistance and oxidative stress, accompanied by an increase in overall energy expenditure[19].
As the predominant flavonoid constituent in an ethanolic extract derived from Mongolian oak (Quercus mongolica) cups, kaempferol significantly lowered fasting blood glucose levels, organ indices for the heart and liver, and serum concentrations of cholesterol, triglycerides, and cardiac malondialdehyde in alloxan-induced diabetic rats[20]. Additionally, kaempferol administration resulted in significant improvements in high-density lipoprotein (HDL) levels and superoxide dismutase (SOD) activity, indicating both metabolic and antioxidant benefits in the diabetic state.
WOGONIN – FLAVONE
In a streptozotocin-induced diabetic mouse model, wogonin significantly downregulated pro-inflammatory mediators, including monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and nuclear factor kappa B (NF-κB). Concurrently, wogonin attenuated renal expression of extracellular matrix components, notably fibronectin, collagen IV, α-smooth muscle actin (α-SMA), and transforming growth factor-beta1 (TGF-β1)[21], indicating its potential to mitigate renal inflammation and fibrosis in the context of diabetic nephropathy.
ISOVITEXIN – FLAVONE
Isovitexin, isolated from Wilbrandia ebracteata, demonstrated potent antihyperglycemic effects in rats, promoting insulin secretion in a manner akin to the activity of oral sulfonylureas[22], which are commonly used in the management of type 2 diabetes.
FISETIN – FLAVONOL
In a streptozotocin-induced diabetic atherosclerosis model in low-density lipoprotein receptor-deficient mice, fisetin treatment effectively mitigated diabetes-induced exacerbation of atherosclerosis. This was achieved through modulation of uric acid, urea, and creatinine levels in both urine and serum. Fisetin also ameliorated morphological damage and fibrosis, reduced the production of reactive oxygen species (ROS), advanced glycation end products (AGEs), and inflammatory cytokines, and attenuated the accumulation of extracellular matrix components[23], thereby offering therapeutic potential in diabetic atherosclerosis.
BAICALIN – FLAVONE
In a type 2 diabetes-induced liver cancer model, baicalin effectively suppressed tumor progression by modulating the METTL3/m6A/HKDC1 axis[24]. Specifically, baicalin influenced the expression of METTL3 (methyltransferase-like 3), m6A (N6-methyladenosine) modification, and HKDC1 (hexokinase domain containing 1), thereby exerting its anti-cancer effects through epigenetic regulation and metabolic pathway modulation.
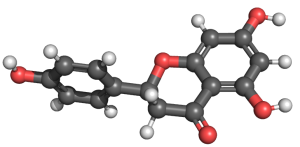
NARINGENIN – FLAVANONE
In a streptozotocin-induced diabetes model in male Sprague Dawley rats, treatment with naringenin in combination with lisinopril resulted in significant improvements in biochemical and urinary parameters[25]. This combination therapy effectively attenuated renal oxidative stress and mitigated renal damage, suggesting a potential synergistic effect in protecting renal function in diabetic conditions.
APIGETRIN – FLAVONE
In streptozotocin (STZ)-induced cell damage in RINm5F cells, treatment with apigetrin effectively inhibited the elevation of intracellular reactive oxygen species (ROS) levels, restored the activity of antioxidant enzymes, and reestablished redox homeostasis[26]. Furthermore, apigetrin significantly suppressed STZ-induced apoptosis and attenuated endoplasmic reticulum (ER) stress, demonstrating its potential as a protective agent against STZ-induced cellular damage.
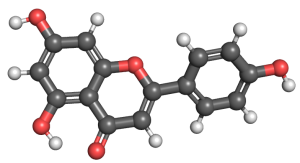
APIGENIN – FLAVONE
Apigenin has been demonstrated to inhibit the proliferation, migration, and angiogenesis of high glucose-induced human retinal microvascular endothelial cells by upregulating miR-140-5p, a known inhibitor of cell proliferation and migration. Additionally, apigenin enhances the expression of PTEN, a tumor-suppressor gene involved in the regulation of cell growth. This action is further complemented by the inhibition of the PI3K/AKT signaling pathway[27], a critical pathway that promotes cell proliferation, growth, and survival while inhibiting apoptosis. These mechanisms collectively underscore apigenin’s potential as a therapeutic agent in retinal pathologies associated with hyperglycemia.
CONCLUSION
The therapeutic landscape of diabetes mellitus is rapidly evolving, with increasing emphasis on plant-derived bioactive compounds as viable adjuncts or alternatives to conventional pharmacotherapy. This article underscores the multifaceted antidiabetic potential of terpenes and flavonoids—particularly those derived from Cannabis sativa and other botanical sources—through diverse molecular and cellular mechanisms. These natural compounds modulate glycemic control, enhance insulin sensitivity, mitigate oxidative stress, suppress chronic inflammation, and attenuate diabetes-associated complications such as nephropathy, atherosclerosis, and even hepatocarcinogenesis. The ability of flavonoids to influence epigenetic regulators and signaling cascades, and of terpenes to exert systemic metabolic and anti-inflammatory effects, highlights their role as pleiotropic agents. Collectively, the emerging preclinical data provides a compelling rationale for further translational studies, including well-controlled clinical trials, to elucidate pharmacokinetics, safety profiles, and synergistic interactions within the phytochemical matrix. As the global burden of diabetes continues to rise, the integration of these natural compounds into evidence-based therapeutic regimens may offer a sustainable and biologically rational approach to disease management and prevention.
[1] Basha RH, Sankaranarayanan C. Beta-caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem Biol Interact. 2016 Feb 5;245:50-8.
[2] Youssef DA, El-Fayoumi HM, Mahmoud MF. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-y receptors. Chem Biol Interact. 2019 Jan 5; 297:16-24.
[3] Jiang N, Zhang Y. Antidiabetic effects of nerolidol through promoting insulin receptor signaling in high-fat diet and low dose streptozotocin-induced type 2 diabetic rats. Hum Exp Toxicol. 2022 Jan-Dec;41:9603271221126487.
[4] US National Library of Medicine. ClinicalTrials.gov. Accessed April 4, 2020.
[5] Aragao, Danielle M O, Lyvia Guarize, Juliana Lanini, Juliana C da Costa, Raul M G Garcia, and Elita Scio. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. Journal of ethnopharmacology 128.3 (2010): 629-633.
[6] Leite, Ana Catarina Rezende et al. Characterization of the Antidiabetic Role of Parkinsonia aculeata (Caesalpineaceae). Evidence-based complementary and alternative medicine: eCAM vol. 2011 (2011): 692378.
[7] Guerra, Joyce Ferreira da Costa et al. Dietary a9ai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. Journal of clinical biochemistry and nutrition vol. 49,3 (2011): 188-94.
[8] Mthembu SXH, Muller CJF, Dludla PV, Madoroba E, Kappo AP, Mazibuko-Mbeje SE. Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules. 2021 Oct 18;26(20):6289.
[9] David SR, Lai PPN, Chellian J, Chakravarthi S, Rajabalaya R. Influence of rutin and its combination with metformin on vascular functions in type 1 diabetes. Sci Rep. 2023 Aug 1;13(1):12423.
[10] de Morais Campos R, Lima LMALL, da Silva AG, Santiago RO, Paz IA, Cabral PHB, Santos CF, Fonteles MC, do Nascimento NRF. Rutin ameliorates nitrergic and endothelial dysfunction on vessels and corpora cavernosa of diabetic animals. Res Vet Sci. 2023 Aug;161:163-172.
[11] Iis Inayati Rakhmat, Euis Reni Yuslianti, Welly Ratwita, Teja Koswara, Nurul Sofiana Mutiadewi. The Effect of Rambutan Honey and Rutin on Decrease Blood Glucose and Increase Streptozotocin-Induced Rat Plasma Insulin. Proceedings of the 13th Annual Scientific Conference of Medical Faculty, Universitas Jenderal Achmad Yani (ASCMF 2022). Advances in Health Sciences Research 16 December 2022.
[12] Wani MJ, Salman KA, Hashmi MA, Siddiqui S, Moin S. Rutin impedes human low-density lipoprotein from non-enzymatic glycation: A mechanistic insight against diabetes-related disorders. Int J Biol Macromol. 2023 May 31;238:124151.
[13] Cai C, Cheng W, Shi T, Liao Y, Zhou M, Liao Z. Rutin alleviates colon lesions and regulates gut microbiota in diabetic mice. Sci Rep. 2023 Mar 25;13(1):4897.
[14] Xianchu L, Ming L. Rutin improves diabetes-induced muscle atrophy in mice. Pak J Pharm Sci. 2023 Jan;36(1):217-221.
[15] Shehnaz SI, Roy A, Vijayaraghavan R, Sivanesan S. Luteolin Mitigates Diabetic Dyslipidemia in Rats by Modulating ACAT-2, PPARα, SREBP-2 Proteins, and Oxidative Stress. Appl Biochem Biotechnol. 2023 Aug;195(8):4893-4914.
[16] Choo, C Y, N Y Sulong, F Man, and T W Wong. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo a-glucosidase inhibition. Journal of ethnopharmacology 142.3 (2012): 776-781.
[17] Li, Zhi-Mei, Ning Liu, Ya-Ping Jiang, Jia-Mei Yang, Jie Zheng, Miao Sun, Yu-Xiang Li, Tao Sun, Jing Wu, and Jian-Qiang Yu. Vitexin alleviates streptozotocin-induced sexual dysfunction and fertility impairments in male mice via modulating the hypothalamus-pituitary-gonadal axis. Chemico-biological interactions 297 (2019): 119-129.
[18]Zhang S, Zhang S, Wang H, Chen Y. Vitexin ameliorated diabetic nephropathy via suppressing GPX4-mediated ferroptosis. Eur J Pharmacol. 2023 Jul 15;951:175787.
[19]Mehanna, Eman T, Norhan M El-Sayed, Amany K Ibrahim, Safwat A Ahmed, and Dina M Abo-Elmatty. Isolated compounds from Cuscuta pedicellata ameliorate oxidative stress and upregulate expression of some energy regulatory genes in high fat diet induced obesity in rats. Biomedicine & pharmacotherapy 108 (2019): 1253-1258.
[20]Yin, Peipei, Yu Wang, Lingguang Yang, Jinling Sui, and Yujun Liu. “Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid, Kaempferol and Their Derivatives.” Molecules: A Journal of Synthetic Chemistry and Natural Product Chemistry 23.5 (2018).
[21] Zheng, Zhi-chao, Wei Zhu, Lei Lei, Xue-qi Liu, and Yong-gui Wu. Wogonin Ameliorates Renal Inflammation and Fibrosis by Inhibiting NF-KB and TGF-B1/Smad3 Signaling Pathways in Diabetic Nephropathy. Drug Design, Development and Therapy 14 (2020): 4135-4148.
[22] Folador P, Cazarolli LH, Gazola AC, Reginatto FH, Schenkel EP, Silva FR. Potential insulin secretagogue effects of isovitexin and swertisin isolated from Wilbrandia ebracteata roots in non-diabetic rats. Fitoterapia. 2010 Dec;81(8):1180-7.
[23]Zou TF, Liu ZG, Cao PC, Zheng SH, Guo WT, Wang TX, Chen YL, Duan YJ, Li QS, Liao CZ, Xie ZL, Han JH, Yang XX. Fisetin treatment alleviates kidney injury in mice with diabetes-exacerbated atherosclerosis through inhibiting CD36/fibrosis pathway. Acta Pharmacol Sin. 2023 Oct;44(10):2065-2074.
[24] Jiang H, Yao Q, An Y, Fan L, Wang J, Li H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m6A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase-3 pathway. Phytomedicine. 2022 Jan;94:153823.
[25]Kulkarni YA, Suryavanshi SV. Combination of Naringenin and Lisinopril Ameliorates Nephropathy in Type-1 Diabetic Rats. Endocr Metab Immune Disord Drug Targets. 2021;21(1):173-182.
[26] Zhang R, Shi J, Wang T, Qiu X, Liu R, Li Y, Gao Q, Wang N. Apigetrin ameliorates streptozotocin-induced pancreatic β-cell damages via attenuating endoplasmic reticulum stress. In Vitro Cell Dev Biol Anim. 2020 Sep;56(8):622-634.
[27] Fu C, Peng J, Ling Y, Zhao H, Zhao Y, Zhang X, Ai M, Peng Q, Qin Y. Apigenin inhibits angiogenesis in retinal microvascular endothelial cells through regulating of the miR-140-5p/HDAC3-mediated PTEN/PI3K/AKT pathway. BMC Ophthalmol. 2023 Jul 6;23(1):302.
No responses yet